The design, development and optimization of process route
1.The design and development of process route
• The assessment of existing route for cost, quality, safety and scalability.
• The design of new route, considering impurity profile, cost, reaction condition, toxic reagent and operational complexity.
• High efficiency for new route development. 2-3 steps per week
• The undesired steps will be accepted by new technologies
2. Process development and optimization
• The development and control strategy of process
• The development of desired process for commercial production and supply
• The process optimization is based on robustness, low cost and quality
• Salt screening, Crystal form screening
• Impurity profile analysis,impurity identification,impurity synthesis
• The research of design space by DoE based on QbD
• The testing and evaluation of process safety(RC1,DSC,TGA,ARC,TSU)
• The application of chemical engineering simulation capabilities on process development and the assessment of equipment risk on process transfer.
The assessment of process safety
• Rapid and accurate measurement of reaction process date,Reaction heat, exothermic rate, heat accumulation, reaction out gassing rate, total amount of out gassing and so on.
• The sample measurement of initial decomposition temperature,heat release, and pressure change.
• The assessment of thermal stability
• The assessment of hazard level of the process
• The assessment of charging rate of material based on the reaction scale and equipment parameters
The comprehensive quality research of API based on QbD
1. The research of CPP
The purpose of Quality risk Management is to confirm the acceptable parameter range(PAR), normal operational range(NOR), and critical process parameter(CPP). Quality risk Management is for every GMP step and need be finished before process validation FMEA provides for an evaluation of potential failure modes for Quality risk Management,DOE strategy is used for complex process conversion, and OVAT strategy is applied for low complex process
2.Impurity and specification Specification and justification based on ICH Q11:
•Define and identification of QTPP and CQA of API
•The impurity research of RSM, intermediate and API based on ICH Q3A
•Development and adjustment analytical method to give justified resolution factor between the actual impurities, potential impurities and carryover impurities in the subsequent step.
•Performing Spike experiment for isomer impurities and process impurities and other by-products
•Researching impurity date of RSM and intermediate and assessing the influence on CQA of API,establishing control strategy for RSM and intermediate
•The report of specification (detailed development data)
3.The research of RSM
•Make recommendations on the definition of registered starting materials in accordance with the guidelines of ICH Q11 and the actual situation of the pharmaceutical authorities in different countries;
•According to the physical properties of the registered starting material, process stability, molecular complexity, etc. to help customers define the appropriate registered starting material
•Systematic research to register impurities in starting materials,such as analogs, precursors, solvents and metals, to determine appropriate standards and control strategies
Process validation
Based on ICH Q7(GMP),in the manufacture of API,person,equipment,material,method and condition will be validated to help ensure that APIs meet the requirements for quality and purity.
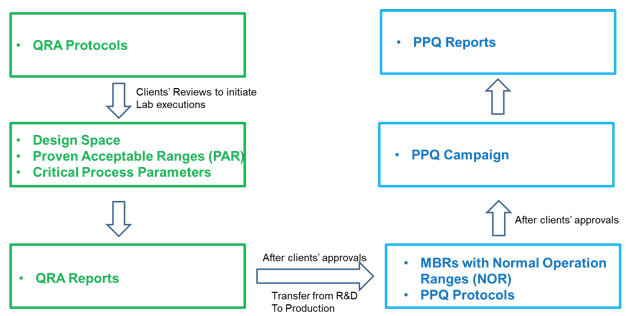
Validation process
Process validation documents:process report, method validation report, batch production record,process validation plan :
• Validation cannot start without QA permission
• Process Validation batches generally consists of 1 to 2 pre-validation batches and 3 process validation
• Stability test of Process validation batch
• Process validation report
Product Lifecycle Management
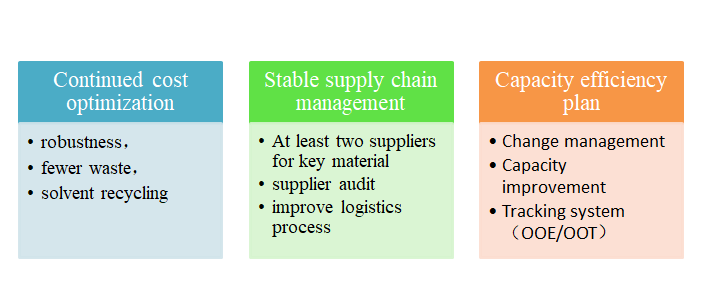
Second generation
process development
•Continued process development,improvement safety,quality and cost by new route and new technology
•The quality of RSM and intermediate of second generation process is same
with previous process
•The re-validation of RSM for new process
•The assessment registration window and definition development strategy
with customer to ensure the second generation process is better.